Axe 2 : Glycomolecule and cell growth
Axe 2 : Glycomolecule and cell growth
Responsables : Arnaud Lehner (PR) & Jean-Claude Mollet (PR)
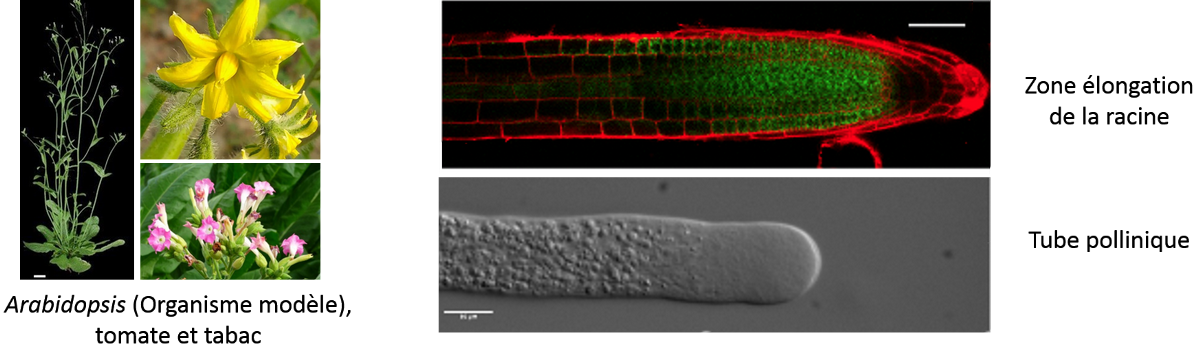
Our project aims to understand the biosynthesis and remodeling of pectin that are known to modulate the cell wall properties, on different aspects of plant cell growth such as elongation, shape, signaling and adhesion. We are currently focusing our research to 1) develop new tools to image the plant polysaccharides in a context of cell growth and 2) to understand the role of pectic polymers in cell-cell adhesion. We mainly focus our work on two models of elongating cells: the elongation zone of the root and the pollen tube.
RG-II imaging
Rhamnogalacturonan II (RG-II) is a complex pectic sub-domain of homogalacturonans. Studying RG-II function and location is challenging because mutants in RG-II biosynthesis are lethal and assays by different groups to obtain RG-II specific antibodies required for immunocytochemistry approaches have failed. Here, we aim at developing new imaging technic to visualize RG-II polymer in the cell wall. The so-called click-chemistry methodology is suitable for the investigation of RG-II localization as, in fact, after metabolic incorporation of Kdo-azide (Dumont et al., 2016, Ropitaux et al., 2022) we can monitor RG-II deposition during cell growth. We will now develop high-resolution imaging using CLEM (Correlative Light Electron Microscopy) and STED (Stimulated Emission Depletion) to have access to the nanoscale distribution of RG-II polysaccharides after coupling to a fluorescent tag.
Pollen tube growth and Adhesion
We are also interested in understanding the role of the cell wall of pollen tubes, rapid tip-expanding cells (Mollet et al., 2007), in cell growth, adhesion and signalling within the female tissues during sexual plant reproduction. We have developed an in vitro adhesion assay, which mimics the female transmitting tract, using pectin-enriched extracts isolated from Arabidopsis pistils and leaves to create an artificial matrix as it was developed for lily pollen tubes (Park et al., 2000; Mollet et al., 2000). Using this in vitro adhesion assay, Arabidopsis pollen tubes adhere and grow on the surface of the matrix. In this project, we are aiming to investigate the minimal structure of the pectin that is required to promote pollen tube adhesion and the effect of this adhesion event on the cell wall remodelling and intracellular machinery.
Projets :





 Adresse : 1er étage Bâtiment CURIB, Place E. Blondel, UFR Sciences et Techniques - Université de Rouen Normandie, F-76821 Mont Saint-Aignan CEDEX France
Adresse : 1er étage Bâtiment CURIB, Place E. Blondel, UFR Sciences et Techniques - Université de Rouen Normandie, F-76821 Mont Saint-Aignan CEDEX France Téléphone Secrétaire administrative : +33 (0)2 35 14 63 56
Téléphone Secrétaire administrative : +33 (0)2 35 14 63 56 E-mail Secrétaire administrative :
E-mail Secrétaire administrative : 

